The Neutrom
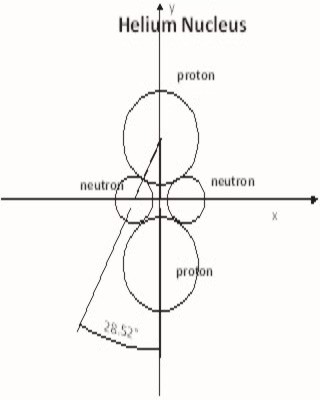
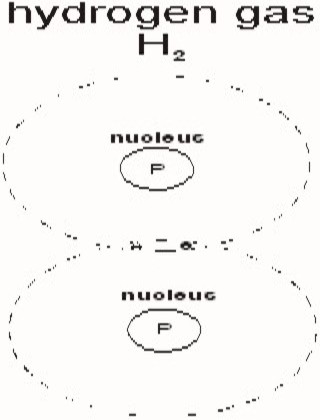
So when it comes down to the neutron where do we start? What is obvious is that the neutron is the binding force or glue that holds a nucleus together. If there are fewer neutrons then protons it's a molecule. The positively charged protons repel each other similar to two north poles in a magnet. Normally two protons repel each other and move away to form two different atoms. Consider H2 gas.
The two hydrogen atoms are tied at the outer limits by the sharing of the two electrons, while the centers are repelled away from each other by the two protons. Now if the proton could be bound together, we would have a Helium atom. Oh that is right the periodic table doesn't tell us that we are short two neutrons. Without these neutrons there is nothing to bind the nucleus together and we have two atoms of H2 gas. So why is a Helium atom different then two hydrogen atoms? The answer is that the helium atom has the binding force or glue of two neutrons. Without this binding force the Nucleus would break apart. In general the number of Neutrons in a Nucleus must be equal to or greater the number of Protons.
The second key function of a Neutron is to determine the outcome of every reaction. If the total number of Neutrons is less then the total number of Proton the outcome of the reaction is a molecule, bound together by the sharing of Electrons in the outer layer while the Protons are
Why a Molecule not an Atom
So how would you combine H2 and O2 to create Ne2? This is the question. Consider water
2H2 + O2 = H2O
Why not
2H2 + O2 = He2
2H2 (1e, 1p) + O2 (8e, 8p) = Ne2 (10e, 10p). It does appear to balance. Oh the neutrons?
2H2 (1e, 1p, 0n) + O2 (8e, 8p, 8n) = 2H2O (10e, 10p, 8n)
This molecule is short 2 neutrons to be Neon Ne (10e,10p,10n).
Fusion
Now we come down to the most misunderstood fact among nuclear physicists. Most physicists believe, that the holy grail of free energy is fusion. In fact fusion will not produce energy, it will consume energy. Take these two facts together to produce Ne2 from H2 and O2 one would add the right amount of energy and two neutrons. So where would, we get the Neutrons? How about heavy water D2O, deuterium oxide. Also we would need a machine which would add the required energy. Shall we call this device an atomizer or maybe a takamat. So lets put this theory to the ultimate test? I am an engineer and inventor so I am sure I can come up with a crude atomizer which may do the job. But I do not have access to heavy water, it is a controlled substance.
Just the same lets start with heavy water and convert it into Neon, with this equation.
2D2O + E (energy) = Ne2
2{D2 (1e, 1p, 1n) + O(8e, 8p, 8n)} + E (energy) = Ne2 (10e, 10p, 10n)
When we combine atoms to create a larger atom (fusion), it consumes energy. When we use particles to strip an atom down, it releases energy. For some reason nuclear physicists do not seem to understand that the law of conservation of energy also applies at the atomic and subatomic level. This is a field of study young physicists should jump into, the thermodynamics of chemistry and nuclear physics. So how is fusion going to provide us with a free source of unlimited power? It never will.
Fission
With these two simple principles let us analyze the A bomb (fission) and the H bomb (fusion). The atomic bomb does use particles to strip atoms down and in the process produces a cascade of additional particles to strip more atoms down releasing a huge amount of energy. The H bomb works a little differently. The H bomb starts out with an atomic bomb (fission) with fusion able materials in near proximity. The hydrogen bomb releases much more energy then an atomic bomb. The question is why.
Fission and Fusion
The H bomb, start with a fission reaction releasing large amounts of energy which the fusion able material absorbs some of that energy and fuses many nucleuses together. But, this is only half the problem. The other half is, are there sufficient neutrons in proximity to be captured in the process to produce a stable larger atom? In fact this is seldom the case. Most fusion reactions are incomplete because the reaction may not capture the required number of Neutrons to make a stable element. The result is called an isotope and will decay over time, back into the original two atoms. The decay releases the same amount of energy that the fusion consumed (conversation of energy). Although this cycle is a zero sum energy cycle, the cycle itself makes the fission in the atomic bomb more efficient. In other words the addition of fusion to a fission bomb makes the fission process more complete, by extending the duration (time) of the reaction. The fusion reaction acts as a catalyst to the fission reaction.
Sun Spots
Consider the sun, which has within the mantel or photosphere both fission, which releases energy, and fusion, which consumes energy, intermingled. The photosphere has a much lower temperature then the corona because the corona has only fission. A large heat difference would be expected between these two. Then when a sun spot boils out of the sun its temperature is much lower then the mantel. This would make the sun spot, a bubble of fusion, which consumes energy lowering its temperature considerably below the surface temperature of the sun.
Lithium Isotope
On a micro scale or atomic level this is what happens, when you fuse helium and hydrogen together to form lithium. In an appropriate atomizer helium and hydrogen will fuse to form a lithium isotope, which over a hundred plus years will decay into its original atoms. A fusion reaction followed immediately by a fission reaction. So why do this? Other then the fact that we can. Well the answer is simple, a new and useful product. The lithium isotope glows, producing a small quantity of light and a small quantity of heat, which will continue for a hundred plus years. I call these minute balls of heat and light "fireflies". You can hold them in your hand with no adverse effect. They also repel each other like two positive charges. This makes them extremely safe in handling and packaging. Do not expect all fusion reactions to be this safe in handling.
H2 + He2 + Energy = 2Li (isotopes)
H2(1e, 1p, 0n) + He2(2e, 2p, 2n) + Energy = 2Li(3e, 3p, 2n) isotope
A lithium isotope that is short neutrons to be a stable lithium atom, since Li = (3e, 3p, 4n). If we added neutrons to this reaction we would form a stable lithium atom.
Now all I need to do is invent the atomizer and put all this theory to the test.
Then I need to complete the periodic table and add the number of stable Neutrons to each element.
I can hardly wait until I hold an atomic "firefly" in my hand. What a pleasure that will be.
To Contact David Graham call 780 450 2574 or email graham777@shaw.ca www.turbogenpower.com/science/fusion.thml